The official publication of the National HBPA – Winter 2021
By Clara Fenger, DVM, PhD, DACVIM; Peter J. Sacopulos, JD; Kimberly Brewer, DVM, MSc; Jacob Machin, MS; Thomas Tobin, MRCVS, PhD, DABT
IS IT POSSIBLE THAT LABS ARE REPORTING IRRELEVANT TRACE-LEVEL IDENTIFICATIONS?
Aminocaproic acid, also known by its brand name Amicar, is a therapeutic medication long used in both human and equine medicine to stop hemorrhage, which it does by stabilizing blood clots. In human medicine, aminocaproic acid is a standard treatment for trauma victims to control and stop hemorrhage and also in surgical patients at risk of hemorrhage to reduce the incidence and severity of hemorrhage.
Consistent with these well-established applications in human medicine, Amicar is often used in equine surgeries that are accompanied by bleeding and hematomas from blunt-force trauma, such as a kick, and for excessive bleeding after surgeries like castration. For many years, Amicar was widely permitted on race day in many jurisdictions as an adjunct bleeder medication, administered with Lasix at four hours prior to post with the goal of reducing the severity of exercise-induced pulmonary hemorrhage (EIPH) in racehorses.
 With the industry moving toward ever-tightening restrictions on therapeutic medications, most jurisdictions have prohibited the use of Amicar on race day, a restriction that has now been in place in most jurisdictions for six or seven years. However, like most other therapeutic medications, when the need arises, aminocaproic acid is still commonly administered to horses in training. For instance, Amicar has still been used in lieu of Lasix to prevent EIPH when breezing between races in order to prevent any possible side effects of using Lasix once or twice a week.
With the industry moving toward ever-tightening restrictions on therapeutic medications, most jurisdictions have prohibited the use of Amicar on race day, a restriction that has now been in place in most jurisdictions for six or seven years. However, like most other therapeutic medications, when the need arises, aminocaproic acid is still commonly administered to horses in training. For instance, Amicar has still been used in lieu of Lasix to prevent EIPH when breezing between races in order to prevent any possible side effects of using Lasix once or twice a week.
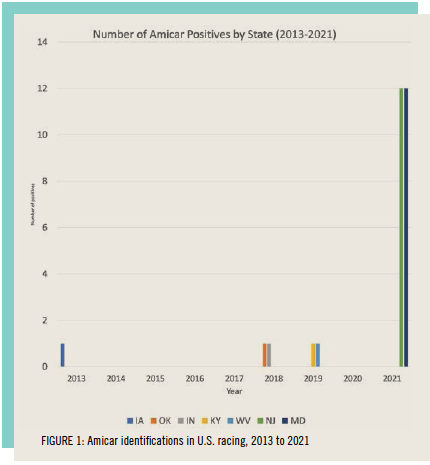
The question then arises as to what the appropriate withdrawal time guideline is for aminocaproic acid administered to horses in training so there is no risk of a trace-level residue detection on race day. This question has been brought into sharp focus by a recent spike in the number of positives called for aminocaproic acid, starting with some well-documented identifications in Maryland and New Jersey racing (Figure 1).
SPIKES IN AMICAR POSITIVES ASSOCIATED WITH LAB CHANGE
Over several weeks in the summer of 2021, a sharp increase in the number of Amicar identifications was reported in racing samples analyzed by Industrial Laboratories in Wheat Ridge, near Denver, Colorado. To our knowledge, some of the first identifications in this sequence of Amicar identifications occurred in Maryland and New Jersey, apparently associated with a change in the equine drug testing contract for these two states from Truesdail Laboratories in Irvine, California, to Industrial Laboratories. This sequence of Amicar identifications has therefore most likely been due to the testing performed by Industrial Laboratories being more sensitive, at least for aminocaproic acid, than the testing being performed previously at Truesdail Laboratories.
This is a classic sequence of events. No two racing chemistry laboratories have exactly the same screening procedures for medication/substance detection in racehorses. This being so, transferring the drug testing contract from one laboratory to another will often serve to make these differences sharply apparent when the laboratory to which the drug testing contract has been transferred is screening for a given substance at a more sensitive level than the previous laboratory. Prior to the Maryland/New Jersey Amicar cluster, the most notable of these laboratory change-driven positives were the 30-plus corticosteroid positives called in the first few weeks after the laboratory contract for New Mexico was switched from UC Davis, where corticosteroid screening was conducted in pooled urine samples, to Industrial Laboratories, where corticosteroid screening is conducted in blood at high sensitivity. Many of these cases were ultimately dismissed.
This difference in screening methodology between laboratories immediately became apparent with the transfer from Truesdail Laboratories to Industrial Laboratories for Maryland’s and New Jersey’s samples. Within days of the transfer, spikes in identifications of multiple therapeutic medications occurred, with the Amicar positives being the most disconcerting. Corticosteroid positive spikes associated with laboratory or threshold changes are common, but the spike in Amicar identifications is a new phenomenon. Investigations have shown that the affected trainers had used Amicar for fast workouts anywhere from three to eight days before the race in which the positive occurred.
A similar spike in Amicar positives also was seen recently without a lab change in Kentucky. Horsemen who had used Amicar in the last work, from five to seven days before racing, were initially notified that they had positives for Amicar. The cases were not pursued for reasons that remain unclear. Since no change had occurred in the use of Amicar among the trainers, it is likely that a change in laboratory screening methodology resulted in this spike.
AMICAR AS A THERAPEUTIC MEDICATION
Aminocaproic acid is structurally related to the amino acid lysine, and it acts to inhibit enzymes that interact with lysine. One of these enzymes is plasmin, a major enzyme that mediates fibrinolysis or, in other words, the breakdown of blood clots. Administration of Amicar enhances the stability of blood clots, which has led to it being approved by the FDA for use in human medicine for the treatment of acute hemorrhage, either traumatic or surgical. Use of Amicar as an adjunct bleeder medication in racehorses predates our understanding of the pathogenesis of EIPH. Clearly, its efficacy as an adjunct bleeder medication comes by reducing the severity of EIPH in horses. By stabilizing a clot once bleeding has occurred, Amicar limits the magnitude of the hemorrhage, rather than preventing the primary EIPH event.
In horses, the dose of aminocaproic acid recommended for use as an adjunct bleeder medication is in the order of 2.5 to 5 grams per horse administered intravenously, a dose well below the dose for humans by weight. Horses exhibit lower levels of fibrinolysis (clot degradation) than humans, so this lower total dose is expected. In a recently published study, the effective Amicar plasma concentration in horses is in the low microgram concentrations, from 3.77 to 7.86 micrograms per milliliter (μg/ml). This same study reportedly indicated no possibility of any pharmacological effect at all below 2 μg/ml.
WITHDRAWAL GUIDELINES
 The Canadian Pari-Mutuel Agency (CPMA) Elimination Guidelines booklet, dated April 2020, documents reported doses of 2.5 and 5 grams of Amicar administered intravenously to horses and suggests an elimination guideline (EG) of 48 hours after administration of either dose. The rate at which Amicar is eliminated by the horse is not discussed, and the threshold levels for plasma and urine are not included in the 2020 CPMA booklet.
The Canadian Pari-Mutuel Agency (CPMA) Elimination Guidelines booklet, dated April 2020, documents reported doses of 2.5 and 5 grams of Amicar administered intravenously to horses and suggests an elimination guideline (EG) of 48 hours after administration of either dose. The rate at which Amicar is eliminated by the horse is not discussed, and the threshold levels for plasma and urine are not included in the 2020 CPMA booklet.
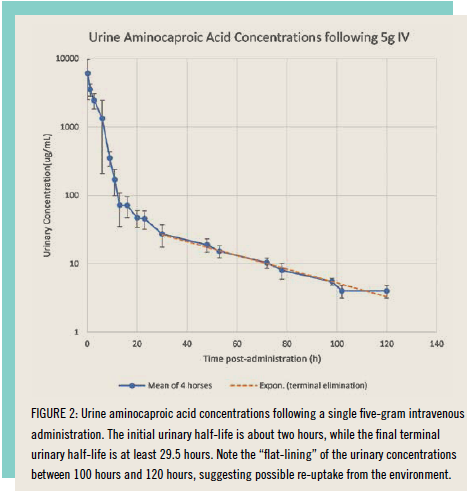
There was, however, a time during the last century when the CPMA was not silent on such matters, and if one reaches back into published CPMA records, one can locate a document dated 1997 in which the plasma and urinary concentrations of aminocaproic acid following these 2.5 and 5 gram per horse intravenous administrations were presented. The intravenous data in plasma cutoff at 12 hours is at about 1 μg/ml, a far from sensitive analytical method by modern 2021 standards. This CPMA method is, however, more than sensitive enough to detect aminocaproic acid in equine urine out to 120 hours or five days post-administration and likely much longer than that given that the mean concentration of aminocaproic acid in these five-day urine samples was in the order of about 5 μg/ml, which is 5,000 nanograms per milliliter (ng/ml), and the terminal urinary half-life of aminocaproic acid at five days post-administration was quite prolonged, as presented in Figure 2, which was replotted from the 1997 CPMA publication on aminocaproic acid.
The 5-gram dose intravenous data immediately answer two questions about aminocaproic detection and elimination in horses. First, the dose of aminocaproic acid is, at 2.5 to 5 grams per horse, relatively large, so an enormous number of molecules has been introduced into the horse for the chemist to detect. The second important question is about the terminal plasma/urinary half-life of aminocaproic acid in the horse. The data in Figure 2 shows that, shortly after administration, the urine Amicar concentration decreases rapidly, but the terminal half-life of aminocaproic acid becomes prolonged beginning at about 24 hours after administration. This can be seen in the graph as the curve becomes flat, decreasing more slowly. While the plasma levels are not known, what is highly likely is that the terminal plasma elimination is similar, very long and very flat. As such, aminocaproic acid is a classic long terminal plasma half-life medication, with microgram per milliliter concentrations persisting in urine for much longer than five days and much lower but similarly persistent plasma concentrations being present in the horse’s plasma for equivalently long periods. It therefore comes as no surprise that if modern 2021 high-sensitivity testing is introduced for aminocaproic acid in either plasma or urine, detection times much longer than five days may be expected, fully consistent with at least one reported plasma identification at eight days after an aminocaproic acid administration.
Based on these data, the next question is what concentrations of aminocaproic acid were being reported in these Maryland, New Jersey and other related Industrial Laboratories-driven identifications. The answer is not clear since Industrial Laboratories apparently elected not to report the estimated aminocaproic acid concentrations in these identifications. However, at this time, the longest reported last time of aminocaproic acid administration is eight days post-administration, by which time the urinary decay curve in Figure 2 is, in lay terms, close to flat, suggesting that detections well beyond eight days postadministration of aminocaproic acid cannot be excluded.
The next question that arises is at what point post-administration the plasma and urinary concentrations of aminocaproic acid become pharmacologically irrelevant. The simplest approach is to look at the recent research showing that any level at or below the 2 μg/ml in plasma is irrelevant and could legitimately be ignored. Alternatively, we can take a far more conservative approach as originally described by professor Pierre-Louis Toutain, a calculation of an irrelevant plasma concentration (IPC). If we take the effective plasma concentration of aminocaproic acid as being 5.82 μg/ml, as reported in the scientific literature, and then divide this figure by a safety factor of 100, the IPC becomes 58.2 ng/ml. Interestingly, the only recent aminocaproic acid identifications for which we have data range between 4 and 46 ng/ml, below this highly conservative IPC for aminocaproic acid.
A LEGAL POINT: A CHANGE IN LEVEL OF DETECTION IS A DE FACTO RULE CHANGE
All changes in the level of detection of foreign substances constitute rule changes for participants and licensees. This is the case whether the change is brought about by a change in the approved testing laboratory or a change in methodology used in testing procedures. In each case, that level of detection change constitutes a de facto rule change.
State regulators, commissions and most rule-making authorities use a negotiated rule-making procedure. The negotiated rule-making procedure has, as one of its key components, notice. By soliciting information and ideas, this process increases the acceptability of the rule or rule change. It also decreases the likelihood of resistance or challenge. Additionally, the negotiated rulemaking procedure often shortens the time necessary to issue a final approved rule. A level of detection change without notice is contrary to the negotiated rule-making process and constitutes a de facto rule.
An example of the negotiated rule-making process is the Association of Racing Commissioners International (ARCI) Model Rules Committee and its procedure and process relative to considering a new rule or a change to an existing rule. The process requires the petitioner to describe the issue or problem and put forward possible solutions. The identification of shareholder groups that are believed to support or oppose the recommendation, as well as the identity of other rules that may require change or would otherwise be affected in the process, is also required. The process includes notice and a merit-based open discussion with input from industry stakeholders.
Conversely, de facto rule changes, such as the sudden level of detection change relative to Amicar, are more commonly the result of an adversary rulemaking procedure. The adversary rule-making procedure deprives the affected parties, including trainers, owners, veterinarians and the public, from facetoface negotiation and exchange of ideas. The adversary rule-making process further deprives the affected parties of shared information and expertise. Finally, rule changes, such as a level of detection change, brought about without notice or negotiated rule-making, often result in successful challenge. However, such challenge, even when successful, most often comes at a high process in time, finances and effort.
Improvements in testing now allow most labs to detect substances and metabolites at thresholds far below what has been seen before. Detecting at picogram per milliliter or one-trillionth of a gram is not uncommon now. At such levels, a level of detection change becomes a de facto rule change, absent notice, and demands the attention of all horsemen. In focusing that attention, regulators must be reminded that substantive due process requires race commissions to establish thresholds and levels of detection that reflect a scientifically accepted connection (i.e., a rational relationship between the substance and its effect on the equine athlete).
The rule-making process is eliminated with limitation of detection changes that constitute de facto rule changes. Rules and regulations are formulated, negotiated, enacted and enforced to ensure compliance from industry participants. The rule-making process allows participants to modify their behavior to comply. To allow limitation of detection change “rules” without notice eliminates the process itself and is detrimental to all shareholders.
CONCLUSION
In summary, a review of the scientific literature shows that aminocaproic acid is a well-recognized therapeutic medication and is FDA-approved and widely used in both human and veterinary medicine for its ability to increase the efficacy of blood clotting. This blood-clotting enhancement effect requires a therapeutic dose, and the standard administration in veterinary medicine is twice a day. A problem in competition horses is that ineffective trace levels of this medication remain detectable in equine plasma and urine for at least eight days or longer post-administration, long after the pharmacological effects have dissipated. It is therefore appropriate to suggest an interim screening limit of detection (SLOD) for aminocaproic acid in post-race plasma and/or urine, and we now recommend a calculated IPC of 60 ng/ml as such an interim SLOD for aminocaproic acid in equine plasma. Any level below 2 ng/ml should be considered a mitigating circumstance. Further, if these recommendations were not to be adopted by regulators, it would be appropriate for regulators to offer a grace period for therapeutic medications in the event of a laboratory change or a methodology change within a laboratory. HJ






