FDA Moves Toward Regulation of Smartphone Apps Used by Medical and Dental Professionals
Michael J. Sacopulos, JD
Waiting to see the Dentist can be trying. There are only so many magazines one can read. Now, Dentists like John Stark in South Jordan, Utah are providing I-Pads to patients in the waiting room. The I-Pads are loaded with dozens of easy to use apps, most of which pertain to dental health.
“Patients love it, it has been pretty amazing how people will play with it,” Dr. Stark said.
According to a 2010 market research report by Research and Guidance, 500 million smartphone users will be using a healthcare application by 2015. Consumers are utilizing these tools almost as quickly as they can be developed. There are apps to allow individuals to monitor their calorie intake for healthy weight management, and apps for physicians to view patients x-rays on their mobile communications device.
On the clinical side Josh Berd, DDS in San Francisco loves using the app “DDS GP”. The application’s library contains a series of presentations that span all topics in dentistry. For example Dr. Berd explains that if a patient needs a filling he can show a video on how cavities are formed.
“If you need a root canal, the app will show you how it is done so you simply don’t have to use your imagination,” Dr. Berd said.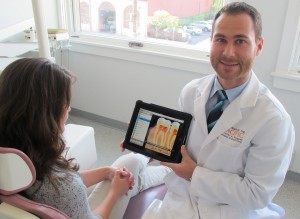
Dr. Stark also prefers using the “DDS GP” app in his practice. Before he would find himself drawing on paper to explain a procedure, now he uses an I-Pad.
“In dental, trying to explain a procedure to a patient is difficult, they don’t have any context to what we are doing,” Dr. Stark said.
But some of the apps used by Dr. Stark and other may soon be regulated by the FDA. The FDA has publically stated they are encouraging the exciting development of new applications that can make healthcare better. On the other side of the coin the FDA also says they have a public health responsibility to oversee the safety and effectiveness of those mobile medical applications that present a potential risk to patients if they don’t work as intently.
“At this time we haven’t received any reports of problems related-specifically to mobile medical applications. We do usually expect to under reporting in devices in general. And perhaps with great, you know, we may see an increase in the number of reports with this increased awareness in the guidance,” Associate Director for External Communications for FDA’s Center for Devices Peper Long said in a recent interview.
The FDA has released a proposal for regulating mobile medical applications or mobile medical apps. The FDA is seeking input on its proposed oversight approach for certain mobile medical applications specific to medicine or healthcare called Mobile Medical Applications, which are designed for use on smartphones and other mobile commuting computing devices. The FDA says they are focusing on only a select group of applications, and will not regulate the sale or general consumer use of smartphone or tablets.
“In order to provide manufactures of such mobile medical applications with the clear understanding for which mobile medical applications the FDA will oversee. The FDA has defined a small subset of mobile and medical applications that may impact performance or functionality of currently regulated medical devices,” Policy Advisor from the Office of the FDAs Center for Devices Bakul Patel said.
Types of Apps to be regulated
The FDA’s definition includes mobile medical applications that are used as an accessory to a medical device, already been regulated by the FDA. One example of this is an application that allows a healthcare professional to make a specific diagnosis by viewing a medical image from a picture archiving and communication system, also known as PACS, on a smartphone or mobile tablet. Another example is an app that calculates a patient’s Glasgow Coma Score based off of physician inputs.
The definition will also include a mobile medical application that can transform a mobile communication device into a regulated medical device by using attachments or sensors. Here an example is an application that turns a smartphone into an ECG machine or an electronic stethoscope to detect abnormal heart rhythms or determine if the patient’s experiencing a heart attack will be considered under this definition.
“We believe this outline approach does not cover the majority of mobile applications and will continue to promote innovation in this new and expanding field,” Patel said.
How you can help
The FDA is encouraging feedback from manufactures, healthcare providers, and other stakeholders in how this proposal will support the balance between promoting innovation and ensuring safety and effectiveness. One can submit an electronic comment to https://www.regulations.gov.
“This is necessary. The FDA, medical and dental communities have to be involved,” Dr. Berd said.
The future
At this time the FDA does not have an enforcement game plan for approaching an app manufacturer if the app does not work up to par.
“I think we will be able to look at it on a case by case basis and we would enforce as it’s appropriate. I don’t think a blanket statement would work in any particular situation including currently regulated medical devices,” Patel said.
“If there is an app that is going to make you more efficient or better connected with your patients or even your staff than why not?” Dr. Berd said.
It is a question that the FDA plans to continue sorting through in the upcoming months.
This work is copyrighted and is the exclusive property of the authors. It may only be used, in whole or in part, with the express written permission of the authors.





